polar vs nonpolar
Most of the atoms have less than eight electrons in their valence shells except the noble gases in the group 18 of the periodic table. The key difference between polar and nonpolar amino acids is that polar amino acids have polarity whereas polarity is absent in nonpolar amino acids.
 |
| The Chemical Bond Ionic Vs Covalent And Polar Vs Nonpolar Schooltube Safe Video Sharing And Management For K12 |
Web Polar vs Nonpolar Covalent Bonds As proposed by the American chemist GNLewis atoms are stable when they contain eight electrons in their valence shell.
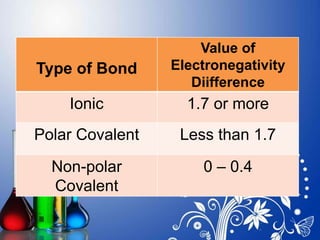
. Examples of Polar and Non-Polar Molecules A molecule may be polar or non. A The electrons in the covalent bond are equally shared by both hydrogen atoms. The difference is 04 which is very small. Nonpolar bonds to read more about bond polarity.
Each kind of atomic relationship requires a different type. The electronegativity difference between bonded atoms is less than 04. Polar vs Nonpolar Solvents. Web Carbon has an electronegativity of 25 while for hydrogen it is 21.
A molecule is polar if one part of it has a partial positive charge and the other part has a partial negative charge. This is a nonpolar covalent bond. The partial positive and partial negative poles are present across the molecule as a result. Web By comparing their EN difference we can say carbon-phosphorus is nonpolar bond.
Thats the short answer to polar vs nonpolar. Web Polar molecules dont mix with the nonpolar barrier like how oil doesnt mix with water in salad dressing. The bond which is formed by sharing a pair of electrons between two atoms is called a Covalent Bond. Technically nonpolar bonding only occurs when the atoms are identical to each other eg H 2 gas but chemists consider any bond between atoms with a.
A compound may possess polar covalent bonds but it may not be a polar compound. However depending on the types of atoms. But because oxygen is small and nonpolar it easily diffuses through the cells in the. Web Molecules come in infinite varieties so in order to help the complicated chemical world make a little more sense we classify and categorize them.
Take an example of water. Refer to polar vs. This happens when there is a difference between the electronegativity values of each atom. Web Atoms are a lot like us - we call their relationships bonds and there are many different types.
The presence of polar functional groups on the chains often enhances chain-chain attractions particularly if these involve hydrogen bonding and thereby. For example consider water and oil. It is a polar compound. Web Polar is a type of covalent bond where atoms share electrons unequally.
Web Solve the math fact fluency problem. These two molecules do not form a solution as they cannot be mixed up. Web Nonpolar and polar molecules exhibit some degree of electronegativity difference between bonded atoms1 Nonpolar bonds. Parasympathetic is the nervous system responsible for your rest and digest responses in times of non-emergencies.
Web Figure PageIndex1 Polar versus Nonpolar Covalent Bonds. The electronegativity values are equal or nearly equal. In this solution water is a polar molecule whereas oil behaves as a non-polar molecule. Theres no element with more negative or positive charge than another side.
Is SeCl 2 a polar or nonpolar molecule. What Makes a Bond Polar. Web Polar bonds are the dividing line between pure covalent bonding and pure ionic bondingPure covalent bonds nonpolar covalent bonds share electron pairs equally between atoms. Atoms of different or same elements come together to form molecules.
Electronegativity values of course. SeCl 2 is a polar molecule because it has an EN difference greater than 04 among atoms. Amino acids can be divided into two groups based on the polarity as polar amino acids and nonpolar amino acids. Web Key Difference Polar vs Nonpolar Amino Acids.
Nonpolar is a type of covalent bond where atoms share electrons equally. Each diagram shows the unsymmetrical shape of the water molecule. Web In a solution a polar molecule cannot be mixed with the non-polar molecule. Therefore they are not stable.
For many molecules the sharing of electrons allows each. In part c the polar covalent bonds are shown as electron dots shared by the oxygen and hydrogen atoms. When in a bond atoms can either share electrons covalent or give them up ionic. Are ionic bonds polar.
Lets go through each. Adaptive and individualized Reflex is the most effective and fun system for mastering basic facts in addition subtraction multiplication and division for grades 2. Web Predicting Polar vs. Both polar bonds and non-polar bonds are two types of covalent bonding between atoms.
Web Ionic Bond Covalent Bond James Bond so many bonds. The atom that holds the. Different ways of representing the polar sharing of electrons in a water molecule. Different atoms show attraction to electrons in various degrees.
Web Main Difference Polar vs Nonpolar Molecules. Web Sympathetic vs parasympathetic the short answer. The reason behind it due to the presence of net dipoles in a polar compound they are asymmetrically arrayed. Web You can then apply these rules to determine if each molecule is polar or nonpolar.
B The fluorine atom attracts the electrons in the bond more than the hydrogen atom does leading to an imbalance in the electron distribution. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. Web Main Difference Polar vs Nonpolar Bonds. What dictates which kind of bond will form.
The degree to which the sharing is equal depends upon the electronegativity. Sympathetic is the nervous system responsible for your fight or flight responses in times of emergencies. Read on for a deep dive into polar vs nonpolar. Web Polar vs Nonpolar.
Andersen shows you how to determine if a bond is nonpolar covalent polar covalent or ioncIntro Music AtributionTitle. The C 5 H 8 monomer isoprene is a volatile liquid. Web Polar Molecules. Nonpolar Covalent Bonds Covalent bonds rely on the sharing of electron pairs between two atoms.
In covalent bonding the electrons are shared between the two atomic species involved instead of a complete giveaway or acceptance of electrons. The CH bond is therefore considered nonpolar. So benzene is a nonpolar solvent. They possess both a partial positive charge and which cannot cancel out.
In a b the polar covalent bonds are shown as lines. Web A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairsThe stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. Web It swells to more than double its size in nonpolar organic solvents like toluene eventually dissolving but is impermeable to water.
 |
| Polar Vs Nonpolar Bonds Overview Examples Expii |
 |
| 9 6 Polar And Non Polar Molecules Physical Science |
 |
| Lesson Explainer Polar And Nonpolar Solvents Nagwa |
 |
| Image Result For Polar Vs Nonpolar Molecules Enlace Covalente Tipos De Enlaces Quimicos Lecciones De Quimica |
 |
| Polar Vs Nonpolar Bonds What Is The Main Difference Psiberg |
Posting Komentar untuk "polar vs nonpolar"